Electrical Synapses |
|
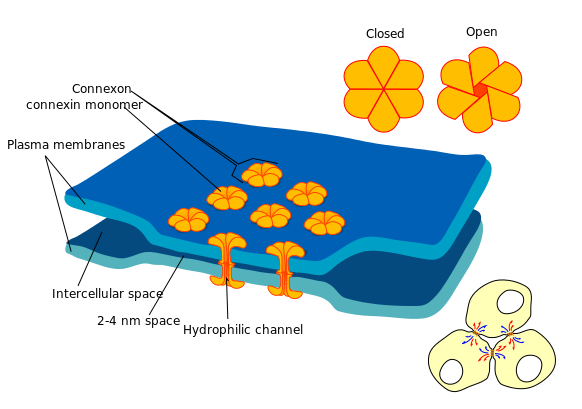
In Electrical synapses a change in membrane potential (in either direction) in one cell is transferred to adjacent cells by current flow through gap junctions between the cells. Electrical synapses are relatively common in the nervous systems of some invertabrates, but are uncommon in the mammalian CNS. They are essential for the function of cardiac ventricular muscle which acts as a functional syncitium because the ventricular action potential passes from one myocyte to the next. Electrical synapses are also found in smooth muscles. In gap junctions the cell membranes of two cells come into very close contact (2-4 nm, compared with the 20 to 40 nm that separates cells at chemical synapses), and are bound together by membrane-spanning proteins called connexins. Connexin molecules provide hydrophilic channels between the two cells and allow current to flow from one cell to the next. Current flow is usually bidirectional, i.e. can occur in either direction acorss the synapse. There are many types of connexin and these function in slightly different ways. Gap junctions couple cells both electrically and chemically, and electrical coupling can be rapid, as in heart muscle. |
In mammals, electrical synapses are found in the brain, mainly between glial cells, but also between one type of horizontal cells in the retina. |
Neuromodulation using gases such as nitric oxide and carbon monoxide Top |
|
A gas such as nitric oxide, produced by the enzyme nitric oxide synthase, can diffuse between cells easily and is removed quickly by chemical conversion into nitrites. First discovered as a messenger between endothelial cells and adjacent smooth muscle, it is now known to also have significant physiological activity at synapses. Spine synapses in the cerebral cortex and hippocampus are sites where it is believed to be produced and diffuse through the adjacent cells and modify the release of neurotransmitters. |
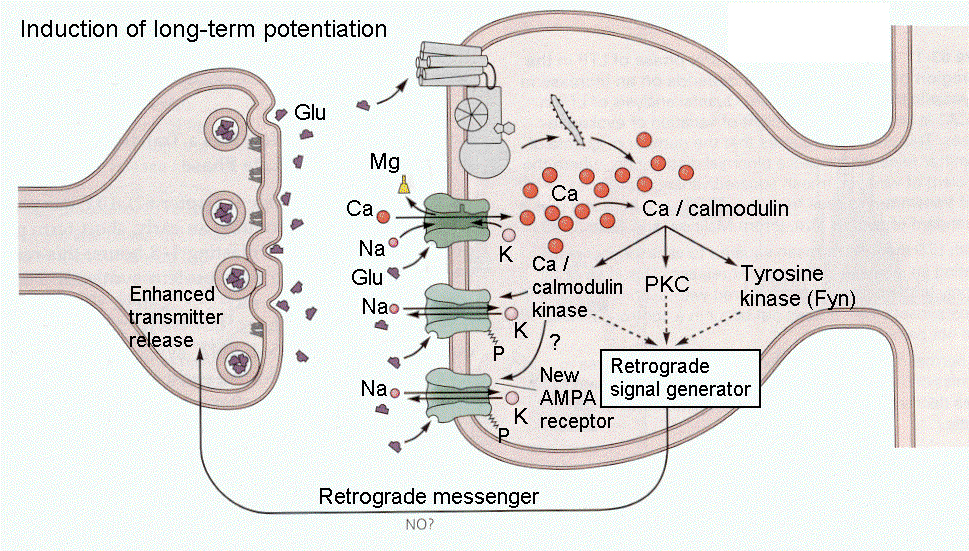
Neuronal nitric oxide synthase is produced post-synaptically at glutamatergic synapses that involve the activation of NMDA receptors. The NMDA receptors allow calcium to enter the neurone and activate the production of cyclic GMP (cGMP) and the synthesis of nitric oxide. This diffusible gas can pass through cell membranes and is believed to be a retrograde transmitter, influencing the presynaptic release of transmitters such as glutamate. |
Presynaptic Actions of Nitric Oxide Nitric Oxide is thought to diffuse into the presynaptic terminal and enhance the expression of the voltage-gated N-calcium channels in the presynaptic neurone. This mechanism is proposed in the pain pathway in the dorsal horn of the spinal cord, as a mechanism for altering pain sensitivity. Nitric Oxide and Synaptic Plasticity In the spine synapses of the cerebral cortex and hippocampus, nitric oxide is believed to be a retrograde messenger that acts presynaptically to trigger the production of cGMP, which in turn influences the release of glutamate. This implies that nitric oxide is involved directly in Long Term Potentiation (LTP) which is a process believed to be involved in the neural basis of memory. More of this in the chapter on the Hippocampus |
|
Carbon monoxide Haem oxygenase 2 (HO-2) is an enzyme that catalyzes the degradation of haem, and produces carbon monoxide. This gas can diffuse from nerve endings and modulate the activity of synapses. |
Less information is available about the role of carbon monoxide than nitric oxide, but some scientists believe that the two gases are co-released in some sites, such as the enteric nervous system, and the brain. |
Interactions between Glia and Neurones Top |
|
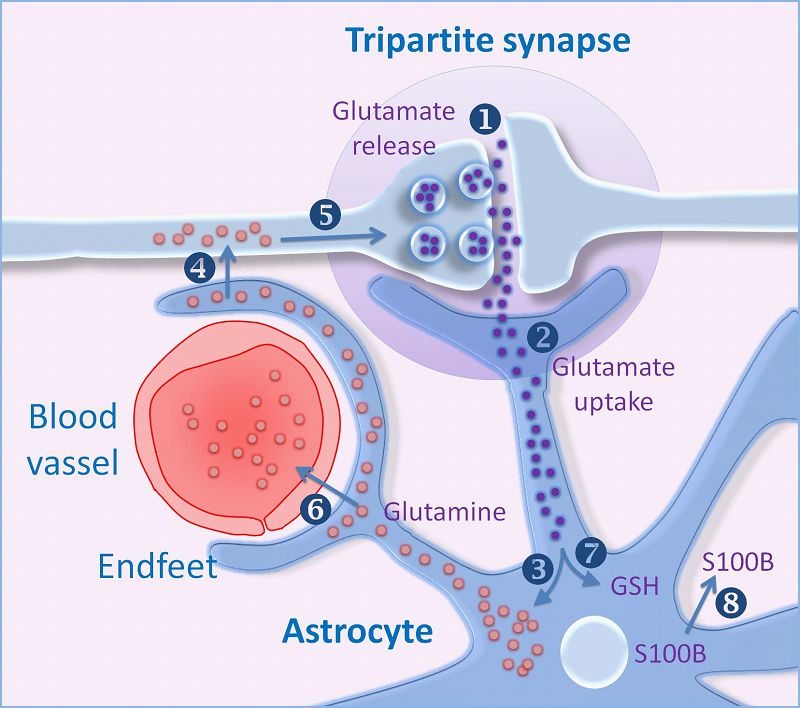
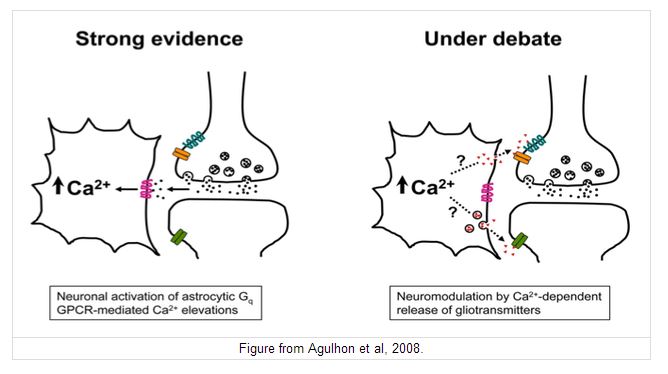
The diagram opposite shows that neurotransmitters released by nerve can act on glial cells - that is known to occur. But in addition it is thought that substances released from glial cells can alter the sensitivity of post-synaptic neurones to neurotransmitters. Glial cells can communicate with each other through their gap junctions, so these mechanisms can have effects that spread away from an active synapse to more distant ones. The diagram (right) indicates a possible scenario for glial-neuronal interactions, and there is increasing evidence for the role of gliotransmitters, e.g. during sleep. Gliotransmitters are neuromodulators that influence the adjacent synapses and there are several contenders including glutamate and ATP.
|
The concept of a 'tripartite synapse' is gaining more support, and indicates that glial cells are not simply supporting cells, buit can actively participate in neural function. In the example on the left, the recycling of glutamine can be seen to involve clearance of glutamate from the synapse, and the formation of glutamine which is the essential component for the formation of glutamate at nerve endings. |
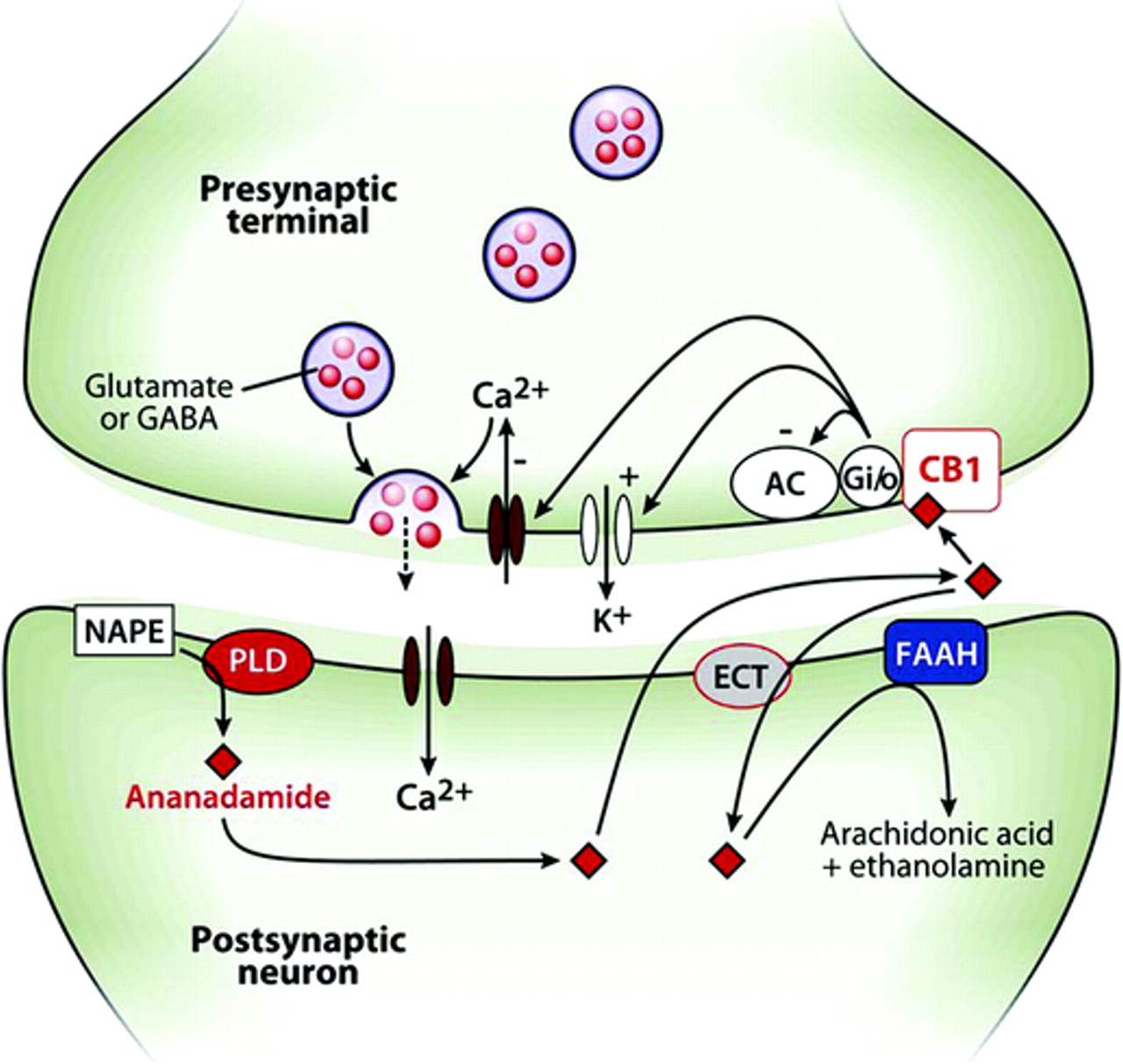
Endocannabinoids are a group of neuromodulatory eicosanoids (derived from membrane lipids), that include anandamide and 2-arachidonoylglycerol (2-AG). They are released as a result of increased calcium levels in the post-synaptic cell, and are known to be involved in the regulation of appetite, mood, pain perception and memory. They are of particular interest because they act as ligands for the cannabanoid receptors, of which there are two main types CB1 and CB2, the former being present in the central nervous system, and the latter in the peripheral nervous system. These receptors are the site of action of cannabis, and operate by a G-protein linked mechanism. The eicosanoids can be taken up by glial cells and converted into arachadonic acd, ethanolamine and glycerol. Anandamide is produced from N-arachidonoyl phosphatidylethanolamine (NAPE) in the cell membrane through cleavage by a phospholipase D (NAPE–PLD). Anandamide is transported into the post-synaptic neurone by an endocannabinoid transporter (ECT) and is broken down by the fatty acid amide hydrolase (FAAH) enzyme, which converts anandamide into ethanolamine and arachidonic acid. Anandamide, generated by the post-synaptic neurone, acts on CB1 receptors in the presynaptic membrane. |
Release of ecosanoids by the post-synaptic neurone may influence the presynaptic release of neurotransmitters. Anandamide may be another retrograde messenger. |