Nociceptors Top |
|
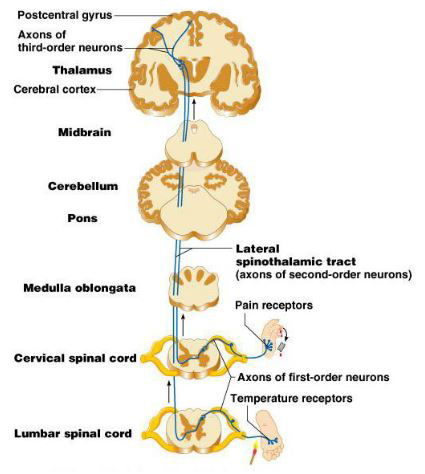
Unmyelinated axons occur in almost all tissues, and the sensory ones generally mediate the senses of pain and temperature. The sensory receptors that respond to injurious stimuli are called nociceptors. Nociception involves two groups of afferent fibres:
These terms ‘slow’ and ‘fast’ point to the speed of conduction of these two fibre groups, and the time take for impulses to travel into the CNS. Both induce flexor and autonomic reflexes. |
Nociceptors respond to injurious stimuli caused by
The mechanism by which nociceptors respond to these intense stimuli requires membrane proteins called TRPV receptors that excite action potentials in the axons by allowing non-specific ion currents to depolarise the free nerve endings.. Transient Receptor Potential Vanilloid channels are a super-family of transient receptor potential (TRP) ion channels, that are selective for calcium and magnesium over sodium ions. Members of the family respond to noxious heat, cold, acid, osmolality and capsaicin, a vanilloid molecule derived from hot red peppers. |
|
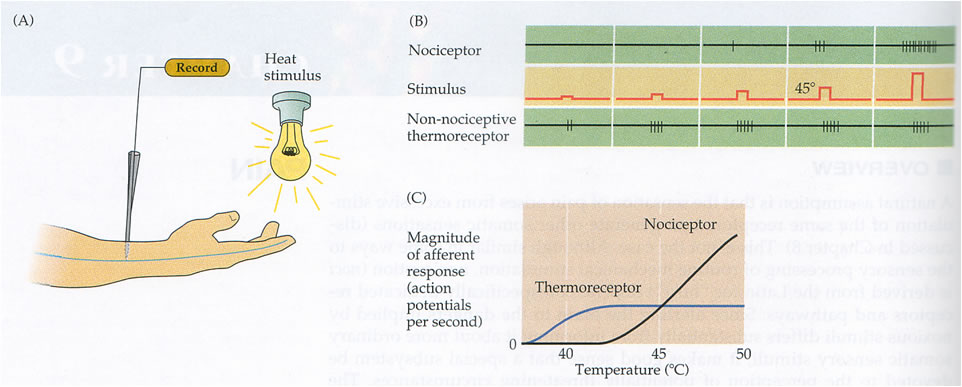
Nociceptors respond to high intensity stimuli. Take temperature as an example (see diagram opposite). The thermoreceptor responds to small increases in temperature BUT the nociceptor only responds to temperatures above 44-46 degrees C – the temperature at which pain can occur and there is evidence of skin injury.
|
|
|
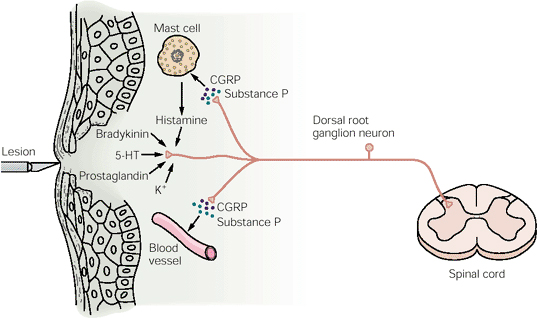
Nociceptive sensory neurones have many branches within the skin and some innervate blood vessels. These branches liberate chemicals that cause vasodilatation and increase capillary permeability. The "axon reflex" is not a proper reflex because there are no synapses in the neural pathway, but noxious stimulation induces redness and tissue swelling because of sensory impulses that release mediators in the blood vessel and cause the "flare". The flare occurs because of vasodilatation (redness) and increased permeability that results in the movement of plama proteins into the extracellular fluid. |
|
Sensitization, Endogenous Algesic Agents and Analgesia Top |
|
|
Sensitization of unmyelinated afferent fibres Sensitization occurs when an unmyelinated nerve sensory ending that is normally responsive only to extreme forces and movements, becomes sensitive to normal forces and movements. One example of sensitization is inflammation: normally nociceptive nerve endings respond only to extreme forces and movements, but, once sensitized, they become much more sensitive and respond to normal movements. In both circumstances the messages they carry give rise to pain. Inflammation In an inflamed joint, a small movement can give rise to pain. IN this condition, the unmyelinated nerve endings can become sensitized, and instead of responding only to large forces and movements, become very sensitive to any movement- because these nerve endings are affected by chemicals produced within damaged tissues, such as
Chemicals with the ability to sensitize the nociceptive nerve endings are known as Endogenous Algesic Agents. Some of the common over-the-counter analgesic drugs act by interfering with the production or action of these agents. |
Endogenous Algesic Agents Bradykinin is a peptide produced by enzymes released from damaged cells acting on a plasma protein, and is found in fluid in inflamed joints. The effects of Bradykinin include :
PGE, PGF2alpha and PGI. They have the following effects:
they increase capillary permeability to plasma proteins, - causing local oedema they are all Algesic Agents Neuropeptides in unmyelinated nerves. Fine unmyelinated afferents contain neuropeptides which can sensitize sensory endings when they are released. These peptides attract cells of the immune system, cause vasodilatation and increased capillary permeability. These neuropeptides include:
|
Analgesic drugs Some analgesic drugs act at peripheral nerve endings. Common analgesic drugs that are bought over the counter act by interfering with the production of prostaglandins that act on the peripheral terminals of unmyelinated axons. These drugs include:
|
|