A neuroanatomist called Rexed looked at the structure of the grey matter of the spinal cord and divided it into columns of cells that were similar to each other in different segments.
His observations were based on the shape and size of the neurones, and he divided the grey matter into 10 (Roman X) layers and 6 of these are in the dorsal horn, numbered I to VI .
The classification has been useful in that the different lamina have neurones with different functions.
Lamina I was the marginal layer or zone.
Lamina II had a gelatinous appearance and is sometimes called the substantia gelatinosa.
Lamina IV and V
had larger cells and this area has also been called the Nucleus Proprius
In Lamina VI another anatomist gave his name to Clarke's column, sometimes called the nucleus dorsalis.
In the ventral horn, lamina VIII and IX contain longitudinal columns of motoneurones
| 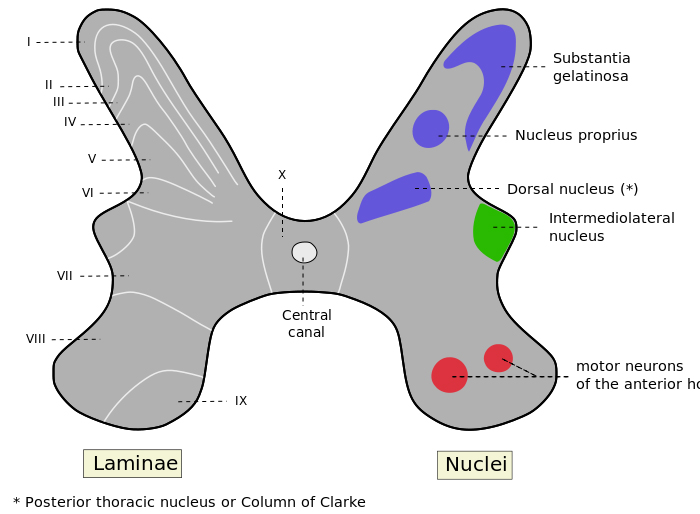 * *
Lamina X is the area around the central canal.
In segments T1 to L2 and the first 4 sacral segments, the grey matter has an intermedio-lateral column of cells which give rise to autonomic neurones
|
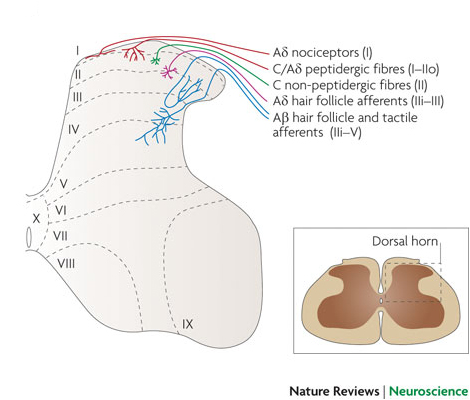
The dorsal horn is concerned with sensory functions, and different types of dorsal root axons originating from the skin and elsewhere synapse in different laminae of the dorsal horn. Some general principles apply: the superficial laminae are concerned with nociceptive sensations, and the deeper laminae process information from low threshold afferents. However some neurones in the depper laminae have inputs from nociceptive and tactile receptors.
Superficial Laminae
Laminae I and II are concerned with processing information from the nociceptive afferents which sense injury and inflammation in their receptive fields and are concerned with pain sensation. The neurones of lamina I, sometimes called marginal cells have long axons that project to the brainstem and thalamus.
Lamina II neurones (the substantia gelatinosa) is concerned with modulating the activity of Lamina I cells. Lamina II cells do not project outside the dorsal horn.
|
 * *
|
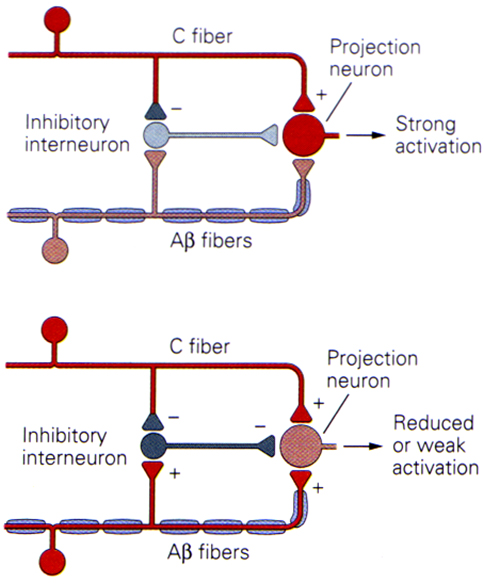 * *
The schematic diagram above shows the concept of 'gating' in the dorsal horn of the spinal cord. It hypothesises that the balance of small and large fibre inputs to ascending neurones of the antero-lateral system modulates the activity transmitted by the ascending pathway.
The diagrams show a projection neurone, possibly equated with the marginal cells of Lamina I that project to the thalamus in the anterolateral system, and the arrangement of afferent neurones that could alter transmission in that pathway.
An inhibitory interneurone is shown (possibly equating to a Lamina II neurone) and the large and small diameter afferent inputs that regulate the activity of that cell can be seen.
The top diagram shows the response to stimulation of small fibres (nociceptors), whereas the bottom indcates the response to simultaneous activation of tactile and nociceptors.
The conclusion is that circuits of this type can modulate the activity of the antero-lateral system, depending on the balance of small and large diameter afferent inputs.
|
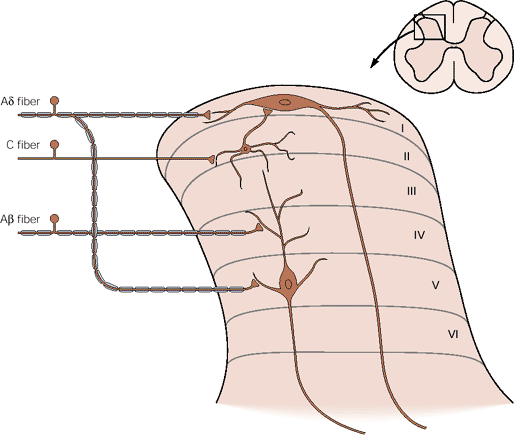 * *
Deeper Laminae of the Dorsal Horn
Lamina IV and V receive inputs from afferents concerned with tactile sensation and with nociception. These cells are called 'wide dynamic range neurones' and project to the brain after crossing the midline and entering the lateral funiculi.
The Gate Theory suggests that the perception of pain depends on the balance of large and small fibre activity entering the dorsal horn, and that large myelinted fibre activity can reduce the onward transmission of nociceptive signals carried by unmyelinated axons.
There is now a lot of evidence that the pain signal can be modulated in the dorsal horn. Stimulation of large afferent fibres, by rubbing the skin, or by electrical stimulation of the skin (TENS) can reduce the perception of pain, and it is a common behaviour to rub an area of skin that has recently been hit by a hard object.
Dorsal column stimulation has been performed in humans using implanted electrodes; these stimulate a collateral of the large afferent neurones, and the impulse that passes antidromically down the cord appears to be able to reduce the perception of pain in ptients with chronic pain conditions. The suggestion is that this mechanism is identical to tht proposed in the Gate Theory.
|